People with talent and determination always find an opportunity to meet their...
Power of DigiProces is based on the people who make up the company Many stories...
Interview with Engineering Manager at DigiProces, Josep Codina Read interview...
Our Lab is continuously improving its equipment and services to ensure we...
DigiProces has been honored with the CEPYME500 recognition for the second...
An advanced solar system to power our Castellar del Vallès plant Loyal to our...
Innovation is a quality inherent to DigiProces that has guided us throughout...
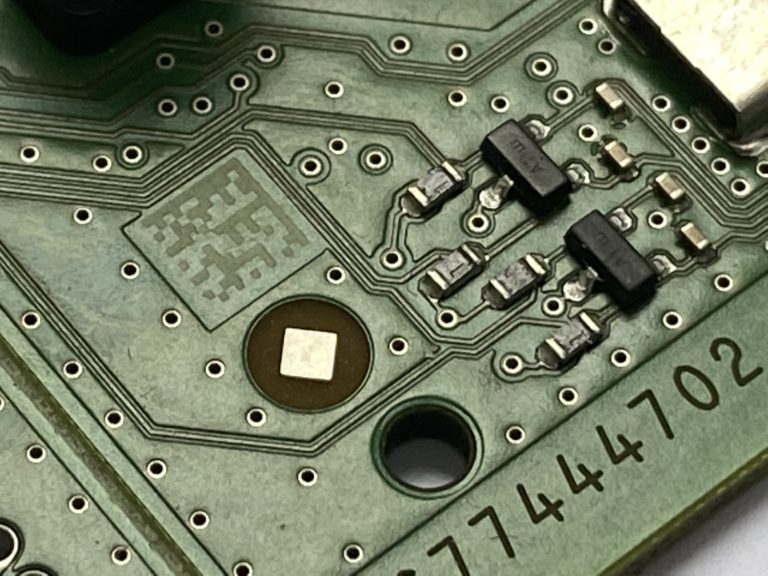
Traceability is the essence of factory digitalization It consists of collecting...
“The best energy efficiency needs electronics The best IoT needs...
The “can be done” attitude of DigiProces towards satisfaction of customer...
Which are the key points to manage operations in a factory like DigiProces At...
What are EMC tests for and why do results need to be validated in a lab EMC...